January 18, 2024
PainTEQ Announces Successful Completion of First In-office LinQ Procedure Under New CMS Billing Code
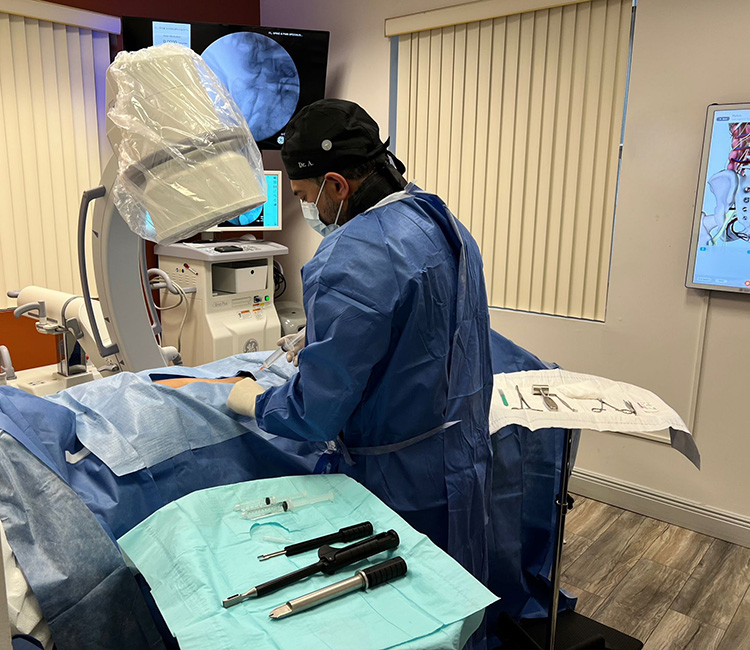
TAMPA, FL – January 18, 2024 – PainTEQ, a leading innovator in minimally invasive sacroiliac (SI) joint dysfunction treatments, has achieved a significant milestone with the successful completion of the first in-office LinQ procedure, now possible under a new billing code finalized by the Centers for Medicare & Medicaid Services (CMS). Nomen Azeem, MD, a respected and internationally recognized interventional pain management physician in the Tampa area, led this pioneering effort using the first-to-market single-use instrumentation of PainTEQ’s posterior SI joint fusion system.
The patient who underwent the LinQ procedure experienced a quick and comfortable recovery, highlighting the procedure’s efficiency and the benefits of an in-office setting. The patient’s positive feedback on the ease and convenience of the procedure underscores its potential to transform SI joint dysfunction treatment.
Dr. Azeem emphasized the importance of this achievement, noting, “Today we were able to prove that this procedure not only can be performed in an office setting but that it was also an uneventful and straightforward experience for both the patient and myself. From the minimal invasiveness of the LinQ implant to the single-use instruments that PainTEQ offers, this is a very viable offering for appropriately trained physicians and properly selected patients.”
PainTEQ CEO Sean LaNeve added, “This is a groundbreaking moment for PainTEQ and for patients suffering from SI joint dysfunction. We’ve now shown that our LinQ procedure can be effectively and safely performed in an office setting, making advanced treatment more accessible and comfortable for patients.”
The LinQ procedure, developed by PainTEQ, represents a major advancement in the treatment of SI joint dysfunction. It is a minimally invasive method that has been traditionally performed in hospital outpatient departments and ambulatory surgery centers. The recent CMS decision to include this procedure in the office setting underlines its safety, efficacy, and the growing need for more accessible treatment options for patients.
PainTEQ’s dedication to clinical excellence and its innovative approach have been key in reaching this significant milestone.
About PainTEQ: PainTEQ was built to bring interventional procedures to the market. Working with pain management specialists to help reduce and eliminate SI joint dysfunction, PainTEQ’s LinQ implant aims to immediately provide clinical benefits to individuals living with incapacitating lower back pain through a minimally invasive outpatient procedure. Learn more at www.painteq.com.